5 Clinical Processes Where Labels Help Maintain Compliance
Labels are an integral part of the workflow for processes throughout a health system, but for these applications, they have a direct impact on compliance.
Medication Management
The Joint Commission medication management standards are both rigorous and challenging. In fact, two categories, medication storage and medication orders, remain frequently scored standards.
Medication Storage
For example, medication storage standards require the drug item to include:
- Medication name, strength, and amount (if not apparent from the container)
- Expiration date when not used within 24 hours
- Expiration date and time when expiration occurs in less than 24 hours
- The date prepared and the diluent for all compounded intravenous admixtures and parenteral nutrition formulas
Medication Orders
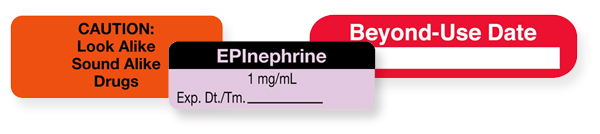
Medication orders have 12 unique elements of performance criteria including one designated as special precautions. The precautions include distinguishing between look-alike sound-alike drug names and highlighting potential medication side effects.
Medication Instruction Labels
Medication instruction labels allow you to record the required storage information details and provide a visual warning for important medication order elements.
Preventing Infections From The Misuse Of Vials
The misuse of single-dose vials caused a Sentinel Event Alert.
Because single-dose vials typically lack preservatives, using them more than once carries substantial risks for bacterial contamination, growth and infection. Although many organizations use single-dose medications more than once as a waste prevention technique. But, any cost savings achieved by preventing waste can quickly be offset by one or more adverse clinical outcomes.
Single- And Multi-Dose Repackaging
In fact, unopened single-dose/single-use vials may be repackaged into multiple single-dose/single-use syringes. But, only with proper labeling.
Similarly, multiple-dose vials can be used again under certain conditions. Once a staff member opens or punctures a multiple-dose vial they must be labeled with a revised expiration date (that is, a beyond-use date).
Medication Labels
For both of these scenarios, a medication label serves as an effective means to meet patient safety and compliance demands. They allow you to record the expiration date and a beyond-use date (which is different from the manufacturer-assigned expiration date) for a single-dose medication, and a 28-day expiration date for multiple-dose vials from the date of opening or puncture.
Sterilization And High Level Disinfection
High-level disinfection (HLD) and sterilization lapses are another common finding of The Joint Commission. Hot spot areas include: transporting soiled instruments safely, transporting non-sharps in a way that cannot lead to contamination, and verifying that the physical parameters of the sterilization cycle have been met.
Transporting Soiled Instruments
Scoring guidelines for transporting soiled instruments reflect the Occupational Safety and Health Administration (OSHA) statement that reusable sharps must be placed in a puncture-resistant red container or one marked “biohazardous.”
Verifying Sterilization
In 2018, nearly 60% of critical access hospitals were found noncompliant to HLD and sterilization process steps. To comply, it’s essential to monitor the presence or attainment of a parameter required for satisfactory sterilization.
Biohazard And Sterilization Labels
For these sterilization and high-level disinfection standards, labels enable compliance.
Biohazard labels that clearly mark containers help healthcare workers operate safely and make that compliance step obvious to surveyors.
Further, autoclave labels provide visual evidence that an item is disinfected and ready for use. The label remains adhered to the item during the sterilization process and it changes color once the proper sterilization level is reached.
Maintaining Medical Gas Systems
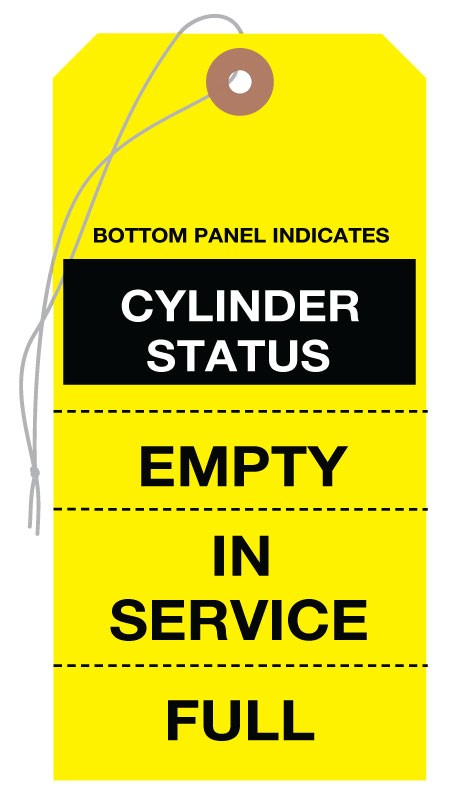
A recent standard change that remains problematic for many hospitals involves storing oxygen cylinders. The Joint Commission requires “those cylinders defined as empty by the organization to be segregated from all other cylinders that are intended for patient use.” This means that full and partially full cylinders can be stored together but empty cylinders must be stored separately.
Labeling cylinders as full, in service or empty and using a racking system, makes them easy to segregate and comply with this standard.
United Ad Label
United Ad Label is an expert at using labels to ensure you meet compliance guidelines. Contact us to learn more.