Medical devices enable healthcare professionals to conduct life saving procedures. But, not when those devices are out of service or not working for any reason. Although it’s impossible to achieve 100% uptime, the inspection, maintenance and safety steps biomedical clinical engineering departments support are essential to properly functioning medical devices. Plus, the medical device labeling they perform when conducting inspections provide key data points that enable safe operating procedures and assist risk management as they proactively work to prevent situations that can result in losses or liability.
Why Is Medical Device Labeling Important?
Medical device labeling informs caregivers about the maintenance regimen, calibration data, safety checks and more for the medical equipment in an operation. This information allows the clinical staff to use medical devices more reliably and elevates their ability to safely treat patients.
When Should You Use Medical Device Labels?
Biomedical clinical engineering departments use medical device labels to record maintenance information, identify out of service equipment, update calibration data, confirm safety checks, enable alarm management, conform to medical device regulations and more. Each of these functions have their own unique needs.
Medical Device Maintenance
Studies show that when medical equipment fails, the most common cause is poor maintenance, planning and management. With the reliance and complexity of medical devices increasing, it puts further emphasis on the importance of both scheduled and corrective maintenance.
Preventive Maintenance
Preventive is one type of scheduled maintenance and is designed to lessen the possibility of medical devices failing at inopportune times. The principle of preventive maintenance is anticipation and incorporates two types of programs, systematic and condition-based maintenance.
- Systematic Maintenance - consists of servicing equipment at regular intervals. No different than examining belts and hoses and changing the oil in a car, systematic maintenance extends the life of the device and can detect premature wear before a breakdown occurs.
- Condition-Based Maintenance - unlike the regular schedule of systematic maintenance, the condition-based maintenance (CBM) strategy monitors the real-time condition of a medical device to determine the appropriate time for maintenance. Those checks include measurements that don’t interfere with operating the device including visual inspection, assessing performance data and scheduling tests. CBM is triggered If any of these indicators show a decrease in performance.
Corrective Maintenance
Corrective maintenance is required when a device isn’t performing at peak level or breaks down when in use. Although the best maintenance programs won’t prevent a medical device from breaking down occasionally, the opposite tactic, running assets until they break, worsens the problem.In fact, it often leads to higher long-term maintenance costs, and when equipment fails unexpectedly, the outage not only disrupts the workflow of the staff using that equipment but can impede the completion of other scheduled maintenance work.
During these maintenance cycles, various information needs to be conveyed to inform the device’s status and ensure downtime is minimized.
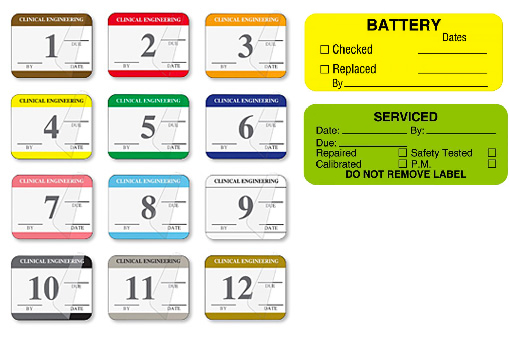 Biomedical Clinical Engineering Maintenance Labels
Biomedical Clinical Engineering Maintenance Labels
There are a variety of labels that biomedical clinical engineering departments use to communicate maintenance status including equipment checked and equipment cleaned labels and tags. In addition, monthly equipment inspection labels help to identify when equipment needs servicing. These items are often customized to include the facility name, department and a contact phone number. You can use the UAL Custom Label Designer to design and order custom labels online.
Medical Device Calibration
Although a medical device enables the diagnosis and ultimate care regimen that wouldn’t be possible otherwise, the prognosis relies on those devices delivering a certainty in measurement. That makes regular calibration an essential piece of medical device management.With repeated use and over a period of time, all equipment begins to degrade affecting accuracy and precision.
Although it varies based on manufacturers guidelines, medical equipment requires calibration on a regular basis. In fact, the FDA mandates that all medical device companies have procedures in place that include instructions and acceptable limits for accuracy and precision. When calibrating a medical device, it should be labeled with the following information:
- The date the device was picked up for calibration
- The name of the staff /company who performed the calibration
- When the next calibration is due
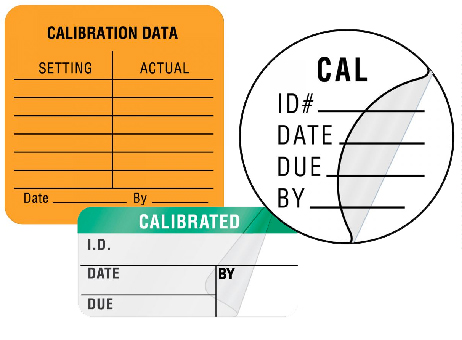 Calibration Labels
Calibration Labels
Calibration labels allow the biomedical clinical engineering departments to record numerous data points to ensure medical equipment accuracy including the last inspection date, next inspection date, specific calibration data and more.
Do you need medical device labeling solutions?
We have hundreds of labels in stock and ready to ship!
Medical Device Safety Precautions
In addition to maintenance and calibration, medical devices require regular safety testing that evaluates their performance and ensures they protect against:
- Electrical Shocks
- Mechanical Hazards
- Unwanted and Excessive Radiation
- Ignition Hazards
- Abnormal Operation and Faults Constructional Defects
In addition, medical devices must comply with IEC 60601-1 3rd Edition, the internationally harmonized safety standard used for all electronic medical devices. Plus, there may be additional requirements for full safety compliance as those set forth by the FDA.
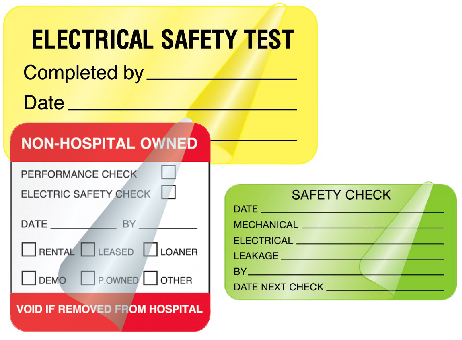 Medical Device Safety Labels
Medical Device Safety Labels
Electrical safety check labels detail when inspections took place and informs the medical staff that the units are secure to use. Plus this type of labeling requirement is often necessary to meet CMS and Joint Commission compliance standards.
How To Label Medical Devices Properly
Knowing how to label medical devices properly will help ensure they remain in service and reduce the potential for putting the patient at risk. As noted above, when a label detaches from a device, it no longer communicates calibration or safety information. Plus, a well functioning device without the proper labeling can cause the medical staff to question the viability of the unit, taking it offline unnecessarily until the device is checked and deemed ready for operation. These additional steps may impact the timeliness of patient care.
Answer these questions to label medical devices properly:
- What material is the label applied to - common medical device materials include metals, polymers and ceramics
- Will the device get exposed to moisture, abrasion, chemicals, or other contaminants?
- Does the label need to remain on the device or should it get removed after a period of time? Inspection and calibration labels need to remain on the device while a maintenance label typically does not
- Will you handwrite information on the label? Does the label run through a printer?
- Does it require a barcode? Defining these specifications will allow you to procure a label that performs effectively.
United Ad Label provides both stock and custom Inspection, Maintenance, Calibration, Safety labels and more that meet the exacting needs of biomedical clinical engineering applications. Plus, this guide can further ensure the labels you purchase fit your needs and operate the way you intended.
Alarm Safety
Alarms alert caregivers to the need for immediate patient care. Yet in actual practice, the results from this simple concept are a mixed bag. For example, false positive alarms are known to be a serious problem. And, so are threats to patient safety due to alarms that are missed, suppressed or not working.
It’s a challenging problem with no easy solution. But, developing a systematic, coordinated approach to clinical alarm system management is a good starting point. An integral step of an effective program is making sure that alarms work.
Medical Device Alarm System Labels
Medical device alarm system labels help health systems track and communicate important safety, maintenance, calibration and inspection information to ensure that alarms sound when needed.
How Do Cleansers Impact Medical Device Labels?
Although medical device cleaning and sanitizing regimens are a standard operating procedure, the importance of those routines have increased due to COVID-19. And regardless of the procedure you use to disinfect a particular device, the cleaning process can cause equipment labels to fail, removing essential maintenance, calibration and safety information.
Using labels and tags designed for the specific cleaning routines a medical device goes through will prevent this problem.
 What Type Of Labels Withstand Medical Device Cleaning Regimens?
What Type Of Labels Withstand Medical Device Cleaning Regimens?
Medical device cleaning typically involves water, detergents, chemical disinfectants or steam and pressure cleaning like autoclave. Each of these routines inflicts stress on medical device labels that may cause them to fail. This increases the importance of selecting a label that fits the regimen the device goes through.
For example, labels that use synthetic films are durable and stand up to moisture, abrasion, chemicals, and other contaminants. Plus, using a clear overlay placed over a label prolongs its life and protects against spills and premature removal. You can learn more about the types of labels that best fit your applications here.
United Ad Label
The medical devices in use throughout a health system are effective when they are operating at peak efficiency. Proper inspection, maintenance, calibration and safety are keys to that operational success. United Ad Label is expert at manufacturing medical device labels that meet the needs of biomedical clinical engineering departments and the exacting requirements of the equipment they maintain. Contact us to learn more.